Quantum numbers: the cosmic barcodes that uniquely identify the properties and states of particles, guiding us through the labyrinth of the quantum world. In this exploration, we embark on a captivating journey to understand the significance and intricacies of quantum numbers, the key to unraveling the mysteries of the microscopic universe.
### **The Quantum Address System**
Imagine particles as celestial residents, each with a distinct cosmic address. Quantum numbers act as the coordinates in this ethereal space, pinpointing the characteristics of particles with unparalleled precision. Let’s delve into the quantum address system and decipher the language of quantum numbers.
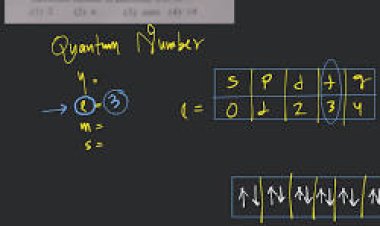
### **Principal Quantum Number (n): The Energy Level**
The principal quantum number, denoted by ‘n,’ serves as the first digit in our quantum address. It determines the energy level of an electron and dictates its distance from the atomic nucleus. Higher ‘n’ values correspond to higher energy levels, leading electrons to orbit farther from the nucleus.
### **Angular Momentum Quantum Number (l): Orbital Shapes**

Now, enter the second digit: the angular momentum quantum number, ‘l.’ This number defines the shape of the electron’s orbital within its energy level. It introduces us to the various orbital shapes—spherical (l=0), dumbbell-shaped (p, l=1), and more exotic configurations. ‘l’ unveils the artistic diversity of electron orbits within an atom.
### **Magnetic Quantum Number (ml): Spatial Orientation**
Zooming further into the quantum address, the magnetic quantum number, ‘ml,’ designates the spatial orientation of an electron’s orbital. Picture electrons navigating within their designated orbitals, each with a unique spatial orientation. ‘ml‘ unveils the intricate dance of electrons around the nucleus.
### **Spin Quantum Number (ms): Intrinsic Spin**

The final digit in our quantum address is the spin quantum number, ‘ms.’ This number reveals the intrinsic spin of the electron, akin to its intrinsic angular momentum. Electrons can have two spin values: +1/2 or -1/2, contributing to the magnetic properties of atoms.
### **Quantum Numbers in Action: Atomic Architecture**
Armed with the quantum address, we can decipher the intricate architecture of atoms. The arrangement of electrons in orbitals follows the principles encoded in quantum numbers, offering a blueprint for understanding atomic structure and behavior.
### **Beyond Atoms: Quantum Numbers in Particle Physics**
Quantum numbers extend their influence beyond the atomic realm. In particle physics, they play a crucial role in characterizing the properties of fundamental particles such as quarks and leptons. The quantum numbers of these elementary particles provide essential information about their nature and interactions.

As we conclude our journey through the quantum realm, the significance of quantum numbers becomes clear—they are the celestial coordinates guiding us through the microcosmos. From determining the energy levels to unveiling the spatial orientations, these numbers serve as the keys to unlock the mysteries of the quantum world. The journey continues, as scientists explore new dimensions and particles, guided by the intricate language of quantum numbers.